A study estimates the percentage of US cancer patients eligible for cutting-edge checkpoint inhibitor drugs has skyrocketed from 1.54% in 2011 to 43.63% in 2018.
But, the treatment has a success rate of only 20-40% and in some cases, it may cause severe auto-immune reactions.
What is the key factor that doctors consider when deciding whether to recommend immunotherapy as a treatment option for their patients?
HLA typing.
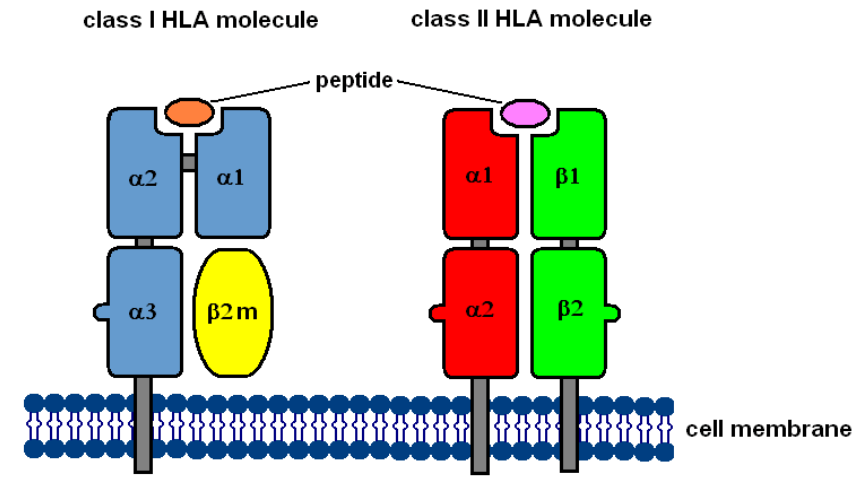
HLA molecules are proteins found on the surface of cells. They help to distinguish between our cells with invaders including bacteria, viruses, etc. HLA molecules present antigens to immune cells for recognition and destruction.
To illustrate, envision your body as a busy city and your immune system as an alert police force. The HLA antigens act as identification cards, enabling the immune system to prevent attacking the body’s own “inhabitants”.
This makes them useful in transplantations, disease diagnosis, drug development, and cancer immunotherapy.
From disease to treatment
When it comes to cancer, HLA typing identifies mutations that produces foreign proteins – recognized as “invaders”.
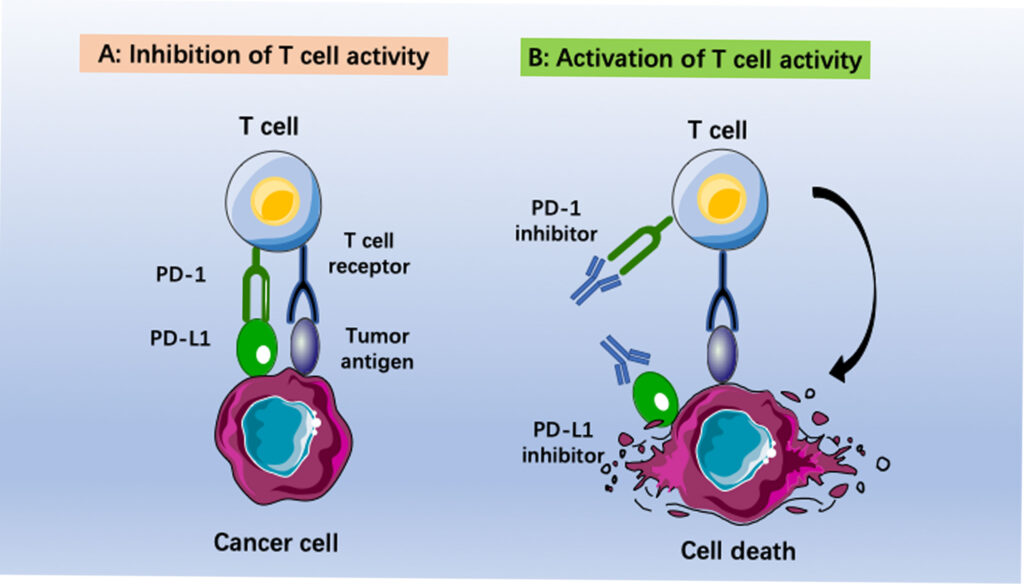
HLA typing predicts whether a patient’s T cells will be able to recognize cancer cells as an “invading entity” and launch an effective immune response when treated with checkpoint inhibitors, allowing doctors to tailor treatment plans to individual patients.
A study in the journal Nature highlights the potential of personalized immunotherapy based on individual HLA profiles in improving the outcomes of cancer treatment. Patients with advanced melanoma who received personalized neoantigen-based vaccines, which were designed based on their individual HLA profiles and tumor-specific mutations, had a significantly higher rate of response and longer progression-free survival compared to patients who received standard immunotherapy.
HLA and Cancer Risk
In addition to its role in cancer treatment, HLA typing provides insights into cancer risk. Certain HLA variants are associated with a higher risk of developing certain types of cancer.
For example, a study states HLA-A*24:02 was associated with an increased risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection.
Another study states HLA-A*01:01 was associated with an increased risk of cervical cancer in patients with human papillomavirus infection.
Understanding these associations can help identify individuals who may benefit from increased screening strategies.
There are several techniques used in HLA typing for immunotherapy. The appropriate HLA typing method is selected based on factors such as accuracy, sensitivity, and throughput.
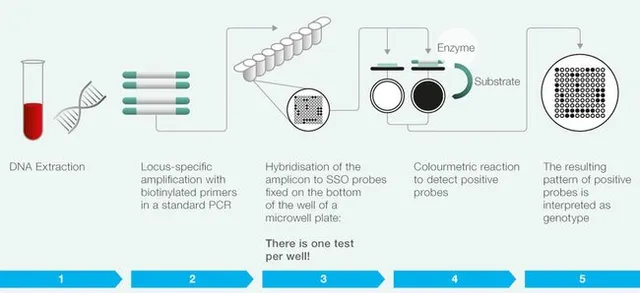
- Sequence-specific oligonucleotide (SSO) typing: This method uses a set of specific DNA probes that bind to the HLA genes and detect specific variations (or alleles) in the DNA sequence. SSO typing is a quick and accurate method for identifying specific HLA alleles in a patient’s DNA, making it a useful tool for predicting a patient’s response to immunotherapy.
- Polymerase chain reaction (PCR) sequencing: This method amplifies the DNA sequences of the HLA genes and then sequences them to identify specific variations in the DNA sequence. PCR sequencing is a more sensitive method than SSO typing and can detect rare alleles that may be missed by other methods.
- Next-generation sequencing (NGS): This is a high-throughput sequencing method that can sequence many genes at once, including the HLA genes. NGS can provide more comprehensive and accurate HLA typing than other methods, making it a useful tool for predicting a patient’s response to immunotherapy.
- HLA tetramer staining: This method uses fluorescently-labeled HLA molecules that can bind to specific T cells in a patient’s blood sample. The binding of the HLA molecule to the T cell indicates that the T cell recognizes and responds to specific HLA-bound peptides, which can be used to predict a patient’s response to immunotherapy.
- ELISpot assay: This is an assay that measures the production of cytokines (molecules that help regulate the immune response) by T cells in response to specific peptides bound to HLA molecules. The ELISpot assay can be used to predict a patient’s response to immunotherapy by measuring the activity of specific T cells.
All of this starts with isolating DNA.
To accurately identify specific variations in HLA genes, high-quality DNA is crucial. The DNA must be intact, free from contaminants, and may require amplification of specific regions using PCR techniques.
And this is where we come in.
We extract DNA from a wide variety of sample types including blood and saliva based on magnetic nanoparticles. The system provides high-quality DNA that is suitable for direct use in most downstream applications including sequencing, PCR, microarrays, etc.
As scientists delve deeper into understanding the intricate relationship between the immune system and cancer, they are discovering ways to target specific HLA variants, leading to improved patient outcomes. Moreover, with the recent advancements in DNA extraction techniques and sequencing technology, HLA typing is becoming more accessible and widely used in clinical settings. By harnessing the power of HLA typing and other advanced technologies, we are set to move closer to a future where cancer can be more effectively diagnosed and treated.
If you’re reading this before May 18, register for a webinar on ‘HLA typing and the significance of high-quality DNA’.
If you’re reading this after May 18, don’t worry we still got you covered. You can find the recording of the session on our YouTube channel.
For any more questions, contact us.