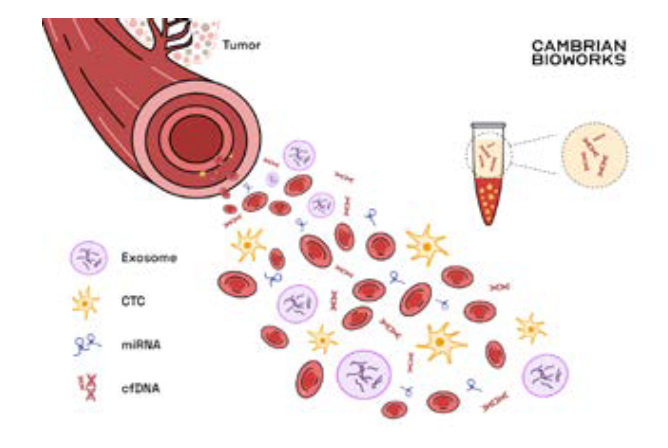
Cell-free DNA (cfDNA) as the name signifies are the bits of DNA present free of cells – in the blood. As a cell dies, it releases its fragmented contents into the blood including diseased DNA among others (exosomes, miRNA, etc.).
This forms the basis of cfDNA analysis diagnosing several conditions.
Why Use cfDNA for Analysis?
- Non-invasive test: cfDNA tests draw blood. This is more convenient and allows repeated biopsies over time.
- Representative of tumor heterogeneity: Single biopsy does not give a complete picture of the genomic landscape. It bypasses driver genes lurking in unsequenced regions. cfDNA varies across different physiological and pathological states. This presents a comprehensive view of the disease intensity and appropriate treatment options.
- Detects patient-specific biomarkers and suitable therapy: cfDNA determines specific genomic alterations. This complements biopsies for real-time molecular monitoring of treatment, detection of recurrence, and tracking resistance.
- Identifies disorders quickly. They use a much smaller sample size and have a quicker turnaround for results compared to tissue biopsies. This allows for better preventive measures.
Does Cell-Free DNA Only Detect Cancer?
cfDNA is a biomarker for cancer diagnostics, prognostics, and therapeutics. Its applications are not limited to oncology:
- cfDNA has a pathophysiological role in autoimmune conditions like HIV, Tuberculosis, and Systemic Lupus Erythematosus (SLE).
- cfDNA detects fetal anomalies – trisomy 21 (Down Syndrome), trisomy 18 (Edward Syndrome), and trisomy 13 (Patau Syndrome).
- cfDNA can identify organ rejection in transplantations.
- cfDNA tracks the safety of gene therapy treatments.
Why is cfDNA Not Used as the Standard Diagnostic Test?
Despite its promising future, the field of cf DNA analysis is young and has limitations.
- Circulating cell-free DNA in the blood has a very short half-life of four to 30 minutes. This makes it hard to examine.
- Sensitivity is low.
- 32%-75% of all testing errors are due to a lack of regulated protocols for sample collection and handling. The wide variability in cfDNA introduces complexity and potential sources of error.
cfDNA Tests in the Market
According to a report published by Global Market Estimates, the Cell-Free DNA (cfDNA) Diagnostics Market is projected to grow from USD 4.9 billion in 2022 to USD 13.1 billion in 2027.
Driving forces
- High prevalence of cancer: WHO estimates that cancer caused roughly 10 million deaths in 2020. This is expanding the demand for prognosis and biopsy devices.
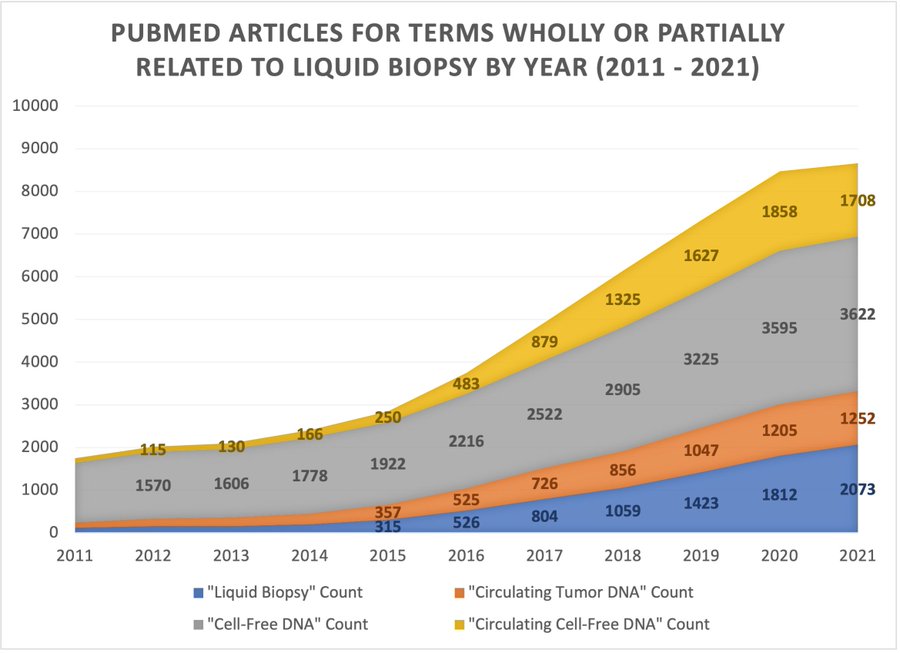
- Increasing research activity associated with liquid biopsy:
- Advancements in Next-Generation Sequencing (NGS): This allows an in-depth cfDNA analysis at a single nucleotide resolution in an economically-viably manner.
What Are the Growth Prospects for Liquid Biopsy Market in India?
Government initiatives aimed at improving cancer awareness, research, and development are anticipated to propel the growth of the liquid biopsy market in India, as per FMI’s market survey.
In India, one in nine people is likely to develop cancer in his/her lifetime. The estimated number of incident cases of cancer in India for the year 2022 was found to be 14,61,427. An increasing number of these cases are compelling the need for advancements in the liquid biopsy field.
Many biotech companies are focusing on R&D for improvements in cancer treatments. Such developments will provide opportunities for market expansion in India.
Some of the top players working in the realm of analyzing cfDNA are:
- Oncophenomics – “We are targeting CTCs to study their sensitivity and resistance to chemotherapy drugs.”
- Medgenome – “The Oncotrack test captures the tumor-derived cell-free DNA and reconstructs the tumor genomic profile without needing to perform a biopsy of the tumor.”
- Strand life science – “We provide end-to-end solutions for genomic testing services in the areas of cancer and rare diseases.”
- Oncodiscover – “OncoDiscover® Liquid Biopsy Test allows for regular monitoring of disease progression and can serve as an early indicator for relapse.”
- DNA Labs – “Our Testing Catalogue includes NIPT, BRCA, Clinical Exome, Liquid Biopsy, and relationship testing.”
- Life cell – “A non-invasive & highly accurate screening test for fetal chromosomal abnormalities.”
We come in the first part of this entire workflow – extracting high-quality DNA from various sample types. We work with some of the major names in the biotechnology and genomics industry; building DNA/cfDNA/mRNA/RNA isolation kits for cell and molecular biology, therapeutics, and genomic sequencing research.
Our products are customizable and can be scaled up according to the customer’s needs. They are suitable for a wide range of applications such as PCR, sequencing, genotyping across molecular diagnostics, oncology research, and academic experiments.
Get free samples now!
To get more information, contact us at support@cambrianbioworks.com or +91 90356 74375
